
Chemistry, 28.05.2020 03:57, arianawelsh123l
"A gas turbine receives a mixture having the following molar analysis: 10% CO2, 19% H2O, 71% N2, at 720 K, 0.35 MPa and a volumetric flow rate of 3.2 m3 /s. The mixture exits the turbine at 380 K, 0.11 MPa. For adiabatic operation with negligible kinetic and potential energy effects, determine the power developed at steady state, in kW." NOTE: the process is NOT isentropic.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:00, raeprince9213
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 23.06.2019 00:00, vanessacox45
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3

Do you know the correct answer?
"A gas turbine receives a mixture having the following molar analysis: 10% CO2, 19% H2O, 71% N2, at...
Questions in other subjects:

Geography, 18.11.2020 05:00

Mathematics, 18.11.2020 05:00

Mathematics, 18.11.2020 05:10


Mathematics, 18.11.2020 05:10


Mathematics, 18.11.2020 05:10

Physics, 18.11.2020 05:10

English, 18.11.2020 05:10


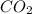 =0.1
=0.1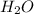 = O.19
= O.19
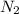 =0.71
=0.71
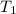 ) mixture receives from turbine =720K
) mixture receives from turbine =720K
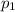 ) mixture receives from turbine =0.35 Mpa
) mixture receives from turbine =0.35 Mpa
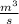
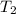 ) = 380K
) = 380K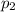 )= 0.11 Mpa
)= 0.11 Mpa
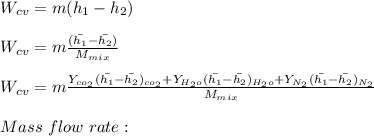
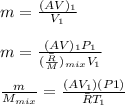
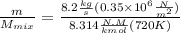
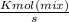
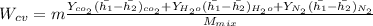
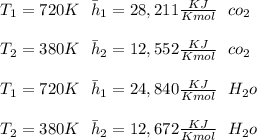
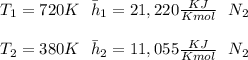
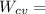 2074.2 KW
2074.2 KW




