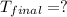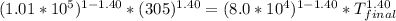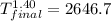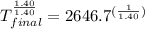On a warm summer day, a large mass of air (atmospheric pressure 1:01 105 Pa) is heated by the ground to 32.0 C and then begins to rise through the cooler surrounding air. (This can be treated approximately as an adiabatic process; why?) Calculate the temperature of the air mass when it has risen to a level at which atmospheric pressure is only 8:00 104 Pa. Assume that air is an ideal gas, with g = 1.40. (This rate of cooling for dry, rising air, corresponding to roughly 1 C per 100 m of altitude, is called the dry adiabatic lapse rate.)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 06:20, Lindsay882
Type the correct answer in each box. balance the chemical equation.__ n203 ➡️ __ n2 +__ o2
Answers: 1

Chemistry, 23.06.2019 06:30, ayoismeisjjjjuan
What is the chemical formula for a compound between li and br? libr li2br libr2 libr3
Answers: 1

Chemistry, 23.06.2019 09:00, msladycie8831
Avogradoa number was calculated by determining the number of atoms in?
Answers: 1

Chemistry, 23.06.2019 19:30, harmonyfern5648
Is the following chemical equation balanced? agno3 + nacl 4agcl + nano3 yes no
Answers: 1
Do you know the correct answer?
On a warm summer day, a large mass of air (atmospheric pressure 1:01 105 Pa) is heated by the ground...
Questions in other subjects:

Social Studies, 15.10.2019 11:50



Advanced Placement (AP), 15.10.2019 11:50

Mathematics, 15.10.2019 11:50


Mathematics, 15.10.2019 11:50


History, 15.10.2019 11:50

English, 15.10.2019 11:50


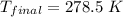
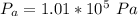
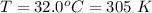
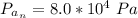
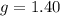
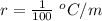
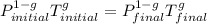
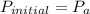 ,
, 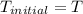 ,
,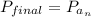 ,
,