
Chemistry, 27.05.2020 04:58, phsycotic121
What is the concentration of the silver ion in silver chromate, Ag₂CrO₄, if its solubility product constant (Kₛₚ) is 1.2 x 10⁻¹². Hint: write the equation first! *
2 points
1.4 x 10⁻⁵
1.1 x 10⁻⁹
1.6 x 10⁻¹²
2.4 x 10⁻¹²

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, cadenhuggins2
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 12:30, hala201490
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 23.06.2019 00:20, destromero
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1

Chemistry, 23.06.2019 08:30, aambitiouss
Of element x has 22 protons, how many electrons does it have
Answers: 1
Do you know the correct answer?
What is the concentration of the silver ion in silver chromate, Ag₂CrO₄, if its solubility product c...
Questions in other subjects:


English, 12.10.2019 09:00

Physics, 12.10.2019 09:00



History, 12.10.2019 09:00



Mathematics, 12.10.2019 09:00


![[Ag^+]=1.3x10^{-4}M](/tpl/images/0666/7571/0a833.png)
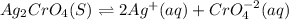
![Ksp=[Ag]^2[CrO_4^{-2}]](/tpl/images/0666/7571/75fed.png)
 due to the dissolution of silver chromate, we obtain:
due to the dissolution of silver chromate, we obtain: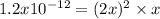
![x=\sqrt[3]{\frac{1.2x10^{-12}}{2^2} } = 6.7x10^{-5}M](/tpl/images/0666/7571/1e8f3.png)
![[Ag^+]=2x=2*6.7x10^{-5}M=1.3x10^{-4}M](/tpl/images/0666/7571/1be44.png)




