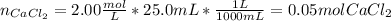Chemistry, 26.05.2020 23:58, graciemartinez9
Cacl2+2AgNO3(aq)-> Ca(No3)2 (aq)+2AgCl (s) if 25.0mL of a 2.00 m CaCl2 solution is used for the reaction shown above how many miles of chloride ions were involved in the reaction? Moles

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, toledanomariap43bxm
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3



Chemistry, 22.06.2019 22:00, Porciabeauty6788
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2
Do you know the correct answer?
Cacl2+2AgNO3(aq)-> Ca(No3)2 (aq)+2AgCl (s) if 25.0mL of a 2.00 m CaCl2 solution is used for the r...
Questions in other subjects:

English, 22.09.2021 15:30



Mathematics, 22.09.2021 15:30

History, 22.09.2021 15:30


Chemistry, 22.09.2021 15:30


Mathematics, 22.09.2021 15:30

Law, 22.09.2021 15:30