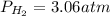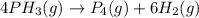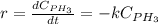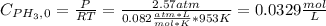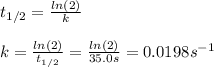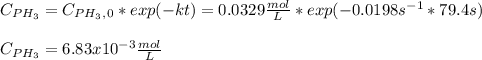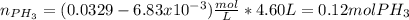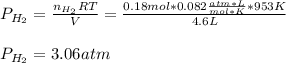Chemistry, 26.05.2020 18:57, eldiamonte
Phosphine, PH3PH3 , is a colorless, toxic gas that is used in the production of semiconductors as well as in the farming industry. When heated, phosphine decomposes into phosphorus and hydrogen gases. 4PH3(g)⟶P4(g)+6H2(g)4PH3(g)⟶P4(g)+6 H2(g) This decomposition is first order with respect to phosphine, and has a half‑life of 35.0 s at 953 K. Calculate the partial pressure of hydrogen gas that is present after 79.4 s79.4 s if a 4.60 L4.60 L vessel containing 2.57 atm2.57 atm of phosphine gas is heated to 953 K

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:10, kaitlynbernatz2778
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 12:30, poopybutt541
Avariable that is not being directly tested during an experiment should be
Answers: 1


Chemistry, 22.06.2019 19:00, georgesarkes12
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2
Do you know the correct answer?
Phosphine, PH3PH3 , is a colorless, toxic gas that is used in the production of semiconductors as we...
Questions in other subjects:

Physics, 27.09.2019 17:30

History, 27.09.2019 17:30




Chemistry, 27.09.2019 17:40



English, 27.09.2019 17:40

Health, 27.09.2019 17:40