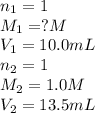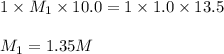Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:50, ajaydonlee
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2


Chemistry, 22.06.2019 20:10, maddie1776
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1

Do you know the correct answer?
A 10.0 mL sample of HNO3 was exactly neutralized by 13.5 mL of 1.0 M KOH. What is the molarity of th...
Questions in other subjects:

Mathematics, 02.04.2021 18:00

Mathematics, 02.04.2021 18:00



Mathematics, 02.04.2021 18:00

Geography, 02.04.2021 18:00

Advanced Placement (AP), 02.04.2021 18:00




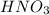 is 1.35 M
is 1.35 M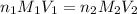
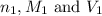 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 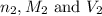 are the n-factor, molarity and volume of base which is KOH.
are the n-factor, molarity and volume of base which is KOH.