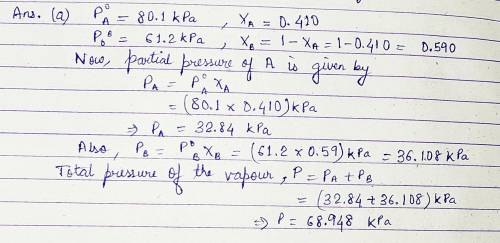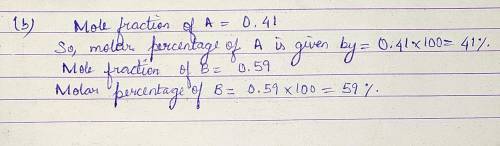Someone pls help me, I need the answer ASAP!!
Pure A (liq) has vapour pressure at 421 K is 80....

Chemistry, 24.05.2020 17:57, Chavens520
Someone pls help me, I need the answer ASAP!!
Pure A (liq) has vapour pressure at 421 K is 80.1 kPa, and pure B (liq) is 61.2 kPa. The two substance form ideal liquid and gaseous mixtures. If the equilibrium composition of a mixture is established, in which the mole fraction of A in the vapour is 0.410, Calculate:
(a) The total pressure of the vapour.
(b) The composition of the liquid mixture.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 00:30, ejuarez2020
When a beta particle is emitted, the mass number of the nucleus a. decreases by one b. increases by one c. remains the same d. decreases by two
Answers: 2

Chemistry, 23.06.2019 06:00, fjsdfj1284
Which change will decrease the number of effective collisions during a chemical reaction? a. adding a catalyst b. increasing the surface area c. decreasing the temperature d. increasing the reactant concentrations e. increasing the volume of the reactants
Answers: 2

Chemistry, 23.06.2019 15:20, brandon0227
Which element below could be an isotope of berylliumsodium-10beryllium-10boron -9carbon-9
Answers: 2

Chemistry, 23.06.2019 16:00, jakebuttone
Afuel has 30.43% nitrogen and 69.57% oxygen. find the molecular formula of the compound if it has a mass of 92 grams per mole a. no b. n2o4 c. no2 d. n4o8
Answers: 1
Do you know the correct answer?
Questions in other subjects:


Mathematics, 01.08.2019 21:30

Mathematics, 01.08.2019 21:30

Chemistry, 01.08.2019 21:30

Social Studies, 01.08.2019 21:30

Mathematics, 01.08.2019 21:30




Mathematics, 01.08.2019 21:30