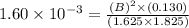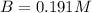At a certain temperature, the equilibrium constant for the chemical reaction shown is 1.60×10−3 . At equilibrium, the concentration of AB is 1.825 M, the concentration of BC is 1.625 M, and the concentration of AC is 0.130 M. Calculate the concentration of B at equilibrium. AB(aq)+BC(aq)↽−−⇀AC(aq)+2B(aq) AB ( aq ) + BC ( aq ) ↽ − − ⇀ AC ( aq ) + 2 B ( aq ) [B] =

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:40, johnnysteeler9934
What is one real world example of a colligative property?
Answers: 2


Chemistry, 23.06.2019 02:40, sherlock19
How can a mixture of salt water be separated into salt and water
Answers: 1
Do you know the correct answer?
At a certain temperature, the equilibrium constant for the chemical reaction shown is 1.60×10−3 . At...
Questions in other subjects:











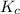
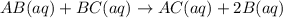
![K_c=\frac{[B]^2\times [AC]}{[BC]\times [AB]}](/tpl/images/0664/6603/29fb9.png)