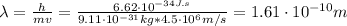Chemistry, 24.05.2020 03:59, Serenitybella
Use the de Broglie's Wave Equation to find the wavelength of an electron moving at 4.5 x 10^6 m/s. Please show your work. Note: h= Plank's constant (6.62607 x 10^-34 J s)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, coopera1744
Find the mass in grams of 1.37x1020 particles of h3po4
Answers: 2


Chemistry, 22.06.2019 16:30, joshua1255
Find the number of moles of argon in 364g of argon.
Answers: 2

Chemistry, 22.06.2019 16:30, montanolumpuy
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
Do you know the correct answer?
Use the de Broglie's Wave Equation to find the wavelength of an electron moving at 4.5 x 10^6 m/s. P...
Questions in other subjects:

English, 21.06.2019 18:00


English, 21.06.2019 18:00

Mathematics, 21.06.2019 18:00

English, 21.06.2019 18:00


Chemistry, 21.06.2019 18:00

Social Studies, 21.06.2019 18:00


Social Studies, 21.06.2019 18:00