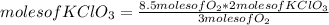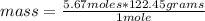Chemistry, 23.05.2020 19:05, gatorlove00
What mass of potassium chlorate is needed to produce 8.50 mol of oxygen? 2KCIO3 →
2KCl +302

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 22:30, darceline1574
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 22.06.2019 22:30, xlebrny7831
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2
Do you know the correct answer?
What mass of potassium chlorate is needed to produce 8.50 mol of oxygen? 2KCIO3 →
2KCl +302...
2KCl +302...
Questions in other subjects:

Mathematics, 19.07.2019 08:10

Health, 19.07.2019 08:10

English, 19.07.2019 08:10

Mathematics, 19.07.2019 08:10




Mathematics, 19.07.2019 08:10