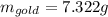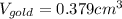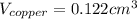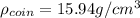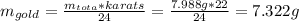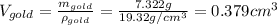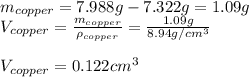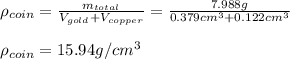Chemistry, 23.05.2020 18:58, fluffylove83
The British gold sovereign coin is an alloy of gold and copper having a total mass of 7.988 g, and is 22-karat gold.
(a) Find the mass of gold in the sovereign in kilograms using the fact that the number of karats = 24× (mass of gold)/ total mass.
(b) Calculate the volumes of gold and copper, respectively, used to manufacture the coin.
(c) Calculate the density of the British sovereign coin.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:40, gabrielolivas59
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 09:00, mercymain1014
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 10:00, emfranco1
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2
Do you know the correct answer?
The British gold sovereign coin is an alloy of gold and copper having a total mass of 7.988 g, and i...
Questions in other subjects:



Mathematics, 27.07.2019 15:30