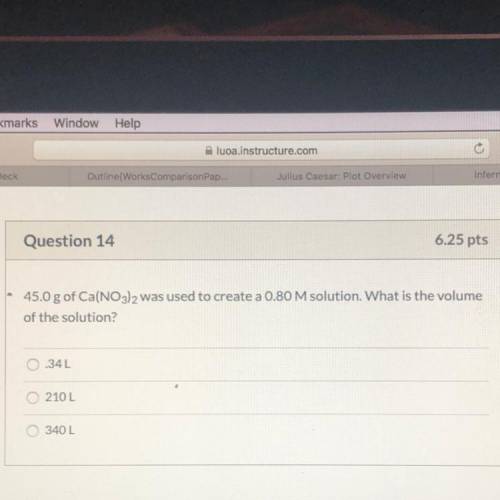
of the solution?


Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, emilyproce
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 16:50, TrueKing184
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1

Chemistry, 23.06.2019 08:00, vetterk1400
Drag each pressure unit with the corresponding number to describe standard atmospheric pressure
Answers: 1

Chemistry, 23.06.2019 09:00, aaronroberson4940
Weight is a measure of: inertia force matter mass
Answers: 1
Do you know the correct answer?
45.0 g of Ca(NO3)2 was used to create a 0.80 M solution. What is the volume
of the solution?
of the solution?
Questions in other subjects:






Social Studies, 25.10.2019 04:43

English, 25.10.2019 04:43