
Chemistry, 22.05.2020 21:57, shanicar33500
(6) A mixture of 40.0 g of oxygen and 40.0 g of helium has a total pressure of 0.900 atm.
What is the partial pressure of each gas?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 00:30, runninglovexoxo
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2

Chemistry, 23.06.2019 01:30, koggebless
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1


Chemistry, 23.06.2019 15:30, dhernandez081
Acontainer holds 6.4 moles of gas. hydrogen gas makes up 25% of the total moles in the container. if the total pressure is 1.24atm. what is the partial pressure of hydrogen
Answers: 3
Do you know the correct answer?
(6) A mixture of 40.0 g of oxygen and 40.0 g of helium has a total pressure of 0.900 atm.
What...
What...
Questions in other subjects:

English, 28.05.2021 06:00

Chemistry, 28.05.2021 06:00

Mathematics, 28.05.2021 06:00

Mathematics, 28.05.2021 06:00


Computers and Technology, 28.05.2021 06:00

Physics, 28.05.2021 06:00


Mathematics, 28.05.2021 06:00

Computers and Technology, 28.05.2021 06:00


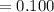 atm
atm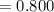 atm
atm 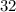 grams per mole
grams per mole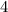 grams per mole
grams per mole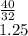
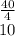
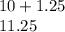
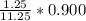 atm
atm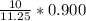 atm
atm



