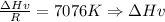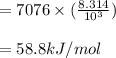Chemistry, 22.05.2020 01:07, jones501324
6.4. The vapor pressure of ethylene glycol at several temperatures is given below:
T (C) 79.7 105.8 120.0 141.8 178.5 197.3
p* (mmHg) 5.0 20.0 40.0 100.0 400.0 760.0
Use a semilog plot based on the Clausius-Clapeyron equation to derive an equation for p* (mm Hg) as a function of T (C). From the plot, estimate the heat of vaporization of ethylene glycol in kJ/mol.
Clausius-Clapeyron equation: ln p* = -Hv/RT + B

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 17:10, macattack6276
What can form as a result of a chemical reaction? what can form as a result of a chemical reaction? compounds isotopes alpha particles beta particles
Answers: 2

Chemistry, 23.06.2019 21:00, Claude7617
Sulfuryl dichloride may be formed from the reaction of sulfur dioxide and chlorine. so2(g) + cl2(g) → so2cl2(g) substance: so2(g) cl2(g) so2cl2(g) δh°f (kj/mol) at 298 k –296.8 0 –364.0 δg°f (kj/mol) at 298 k –300.1 0 –320.0 s°(j/k • mol) at 298 k 248.2 223.0 311.9 what is δg°rxn for this reaction at 600 k?
Answers: 2
Do you know the correct answer?
6.4. The vapor pressure of ethylene glycol at several temperatures is given below:
T (C...
T (C...
Questions in other subjects:

Mathematics, 01.09.2019 14:30

English, 01.09.2019 14:30


Chemistry, 01.09.2019 14:30

Business, 01.09.2019 14:30



History, 01.09.2019 14:30


History, 01.09.2019 14:30


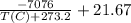
![Exp[\frac{-7076}{T(C)+273.2}+21.67 ]](/tpl/images/0660/5678/69715.png)