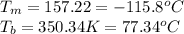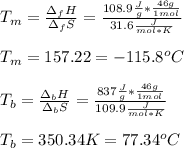Chemistry, 22.05.2020 01:10, questions61
For ethyl alcohol, C2H5OH, the enthalpy of fusion is 108.9 J/g, and the entropy of fusion is 31.6 J/mol •K. The enthalpy of vaporization at the boiling point is 837 J/g, and the molar entropy of vaporization is 109.9 J/mol •K.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, david838843
Iwll give extra points to who gets this for ! what type of reaction is this? ?
Answers: 2

Chemistry, 22.06.2019 03:00, bobbycisar1205
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 04:30, only1cache
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 12:30, AnastasiaJauregui
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1
Do you know the correct answer?
For ethyl alcohol, C2H5OH, the enthalpy of fusion is 108.9 J/g, and the entropy of fusion is 31.6 J/...
Questions in other subjects:


History, 29.08.2019 23:10

History, 29.08.2019 23:10




Mathematics, 29.08.2019 23:10

Computers and Technology, 29.08.2019 23:10