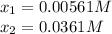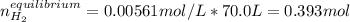Chemistry, 22.05.2020 02:10, Flameking1223
For the reaction
H2(g) + CO2(g) ⇆ H2O(g) + CO(g)
at 700°C, Kc = 0.534. Calculate the number of moles of H2 that are present at equilibrium if a mixture of 0.680 mole of CO and 0.680 mole of H2O is heated to 700°C in a 70.0−L container.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:50, stodd9503
Your roll: experienced electron speech is adressed to: a new "freshman class" of electrons job: write a speech task: you are to pretend that you are giving a speech to a new group of electrons. be sure to mention their placement in an atom, their charge, and their role in chemical bonding (ionic and covalent) be specific!
Answers: 3



Do you know the correct answer?
For the reaction
H2(g) + CO2(g) ⇆ H2O(g) + CO(g)
at 700°C, Kc = 0.534. Calcu...
H2(g) + CO2(g) ⇆ H2O(g) + CO(g)
at 700°C, Kc = 0.534. Calcu...
Questions in other subjects:

Chemistry, 21.08.2020 02:01


Social Studies, 21.08.2020 02:01

Arts, 21.08.2020 02:01

Mathematics, 21.08.2020 02:01

Mathematics, 21.08.2020 02:01

Social Studies, 21.08.2020 02:01

Mathematics, 21.08.2020 02:01

Physics, 21.08.2020 02:01


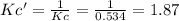
![Kc'=\frac{[H_2][CO_2]}{[H_2O][CO]}](/tpl/images/0660/7360/14ed3.png)
 due to the reaction extent:
due to the reaction extent:![Kc'=\frac{(x)(x)}{([H_2O]_0-x)([CO]_0-x)}=1.87](/tpl/images/0660/7360/2d81b.png)
![[H_2O]_0=[CO]_0=\frac{0.680mol}{70.0L}=0.0097M](/tpl/images/0660/7360/92f9f.png)