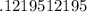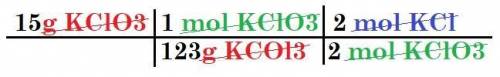Given the following reaction:
2 KCIO3
---> 2 KCl + 302
How many moles of KCl will...

Chemistry, 20.05.2020 23:57, whocares1234
Given the following reaction:
2 KCIO3
---> 2 KCl + 302
How many moles of KCl will be produced from 15,0 g of KCIO3?
moles

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:40, Maryjasmine8001
Salicylic acid is a very important acid. it is used to synthesize the aspirin by treating with acetic anhydride. a 0.2015-g sample of salicylic acid was dissolved in a 100.00-ml volumetric flask, and the solution was diluted to the mark. a 10-ml aliquot of this solution was titrated with standard naoh (0.01130 + 0.2% n) to a phenolphthalein faint pink color end point at 19.81 ml. (a) (calculate the normality of the salicylic acid solution used in the titration. (b) assuming the salicylic acid is pure, what is the equivalent weight of the salicylic acid? practice problems for the final exam (continued) (c) (calculate the inherent error in the determination of the equivalent weight you calculated in part (b). use the following absolute errors in the equipment /glassware when calculating the inherent error. 5.00-ml pipet: + 0.02 ml 100-ml volumetric flask: + 0.08 ml analytical balance: + 0.2 mg 25-ml buret: + 0.03 ml
Answers: 2

Chemistry, 22.06.2019 09:50, revlonknox6
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 22:30, teagan56
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1
Do you know the correct answer?
Questions in other subjects:


Mathematics, 04.02.2021 21:40

Mathematics, 04.02.2021 21:40

Mathematics, 04.02.2021 21:40

Mathematics, 04.02.2021 21:40

Computers and Technology, 04.02.2021 21:40


Mathematics, 04.02.2021 21:40


Mathematics, 04.02.2021 21:40