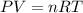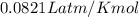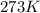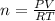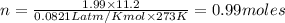Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, electrofy456
What diagram shows the ionic compound of magnesium oxide
Answers: 2

Chemistry, 22.06.2019 14:00, ashlynneboogs0056
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 23.06.2019 00:00, jasmin5285
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1

Chemistry, 23.06.2019 10:30, dreamxette3119
Fill in the blanks for the following statements: the rms speed of the molecules in a sample of h2 gas at 300 k will be times larger than the rms speed of o2 molecules at the same temperature, and the ratio µrms (h2) / µrms (o2) with increasing temperature. a not enough information is given to answer this question b sixteen, will not change c four, will not change d four, will increase e sixteen, will decrease
Answers: 2
Do you know the correct answer?
Which sample would have the same number of molecules as 11.2L of He (g) at 273K and 202kPa?
1...
1...
Questions in other subjects:

English, 05.06.2020 17:57


English, 05.06.2020 17:57

Mathematics, 05.06.2020 17:57

Mathematics, 05.06.2020 17:57


Mathematics, 05.06.2020 17:57

Mathematics, 05.06.2020 17:57

Mathematics, 05.06.2020 17:57

English, 05.06.2020 17:57


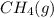 at 273K and 202kPa
at 273K and 202kPa