
Chemistry, 21.05.2020 22:08, rsimmons696
The ph of an aqueous solution at 25.0°c is 10.66. what is the molarity of h+ in this solution? the ph of an aqueous solution at 25.0°c is 10.66. what is the molarity of h+ in this solution? 4.6 à 10-4 3.3 2.2 à 10-11 1.1 à 10-13 4.6 à 1010

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, mayamabjishovrvq9
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 09:30, psychocatgirl1
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone, due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 13:30, kkingstone7062
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1

Chemistry, 22.06.2019 15:00, NatalieKnows
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1
Do you know the correct answer?
The ph of an aqueous solution at 25.0°c is 10.66. what is the molarity of h+ in this solution? the...
Questions in other subjects:

Health, 29.01.2021 22:50

Health, 29.01.2021 22:50


English, 29.01.2021 22:50

Mathematics, 29.01.2021 22:50



Mathematics, 29.01.2021 22:50



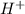 in this solution is
in this solution is 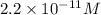
![pH=-\log [H^+]](/tpl/images/0659/9285/37e81.png)
![10.66=-\log[H^+]](/tpl/images/0659/9285/4a0df.png)
![[H^+]=10^{-10.66}](/tpl/images/0659/9285/0b8ff.png)
![[H^+]=2.2\times 10^{-11}](/tpl/images/0659/9285/b8988.png)





