
Chemistry, 21.05.2020 18:00, NathanaelLopez
Carbon disulfide is prepared by heating sulfur and charcoal. The chemical equation is ; =9.40 at 900 K How many grams of CS₂(g) can be prepared by heating 13.8 mol S₂(g) with excess carbon in a 8.60 L reaction vessel held at 900 K until equilibrium is attained?
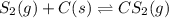

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:10, dhailyortegacampa131
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 11:00, 21villalobosjabez
Which type of fossil does this image depict?
Answers: 1

Chemistry, 22.06.2019 12:30, Svetakotok
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 12:50, martinez6221
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1
Do you know the correct answer?
Carbon disulfide is prepared by heating sulfur and charcoal. The chemical equation is ; =9.40 at 900...
Questions in other subjects:






English, 25.07.2019 13:30


Advanced Placement (AP), 25.07.2019 13:30








