
Chemistry, 21.05.2020 03:09, babygirl62716
In a study of the conversion of methane to other fuels, a chemical engineer mixes gaseous methane and gaseous water in a 0.669 L flask at 1,020 K. At equilibrium, the flask contains 0.276 mol of CO gas, 0.207 mol of H2 gas, and 0.231 mol of methane. What is the water concentration at equilibrium (Kc = 0.30 for this process at 1,020 K)? Enter to 4 decimal places. HINT: Look at sample problem 17.7 in the 8th ed Silberberg book. Write a balanced chemical equation. Write the Kc expression. Calculate the equilibrium concentrations of all the species given (moles/liter). Put values into Kc expression, solve for the unknown.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, bettybales1986
What effect does nuclear radiation have on atoms?
Answers: 1

Chemistry, 22.06.2019 09:50, bridgetosanders
What are four significant sources of ghgs that come from wostem washington?
Answers: 2


Chemistry, 22.06.2019 12:30, murtaghliam1
Word equation for k(s) +h2o(l) yield koh (aq) + h2
Answers: 3
Do you know the correct answer?
In a study of the conversion of methane to other fuels, a chemical engineer mixes gaseous methane an...
Questions in other subjects:


History, 21.04.2020 17:58

Mathematics, 21.04.2020 17:58


Geography, 21.04.2020 17:58

Mathematics, 21.04.2020 17:58

Mathematics, 21.04.2020 17:58


History, 21.04.2020 17:58


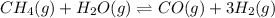
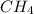 , [
, [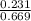 M = 0.345 M
M = 0.345 M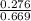 M = 0.413 M
M = 0.413 M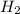 , [
, [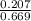 M = 0.309 M
M = 0.309 M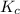 is expressed as:
is expressed as: ![K_{c}=\frac{[CO][H_{2}]^{3}}{[CH_{4}][H_{2}O]}](/tpl/images/0658/4057/27a18.png)
![\Rightarrow [H_{2}O]=\frac{[CO][H_{2}]^{3}}{[CH_{4}].K_{c}}=\frac{(0.413)\times (0.309)^{3}}{(0.345)\times (0.30)}= 0.1177](/tpl/images/0658/4057/4d76c.png)




