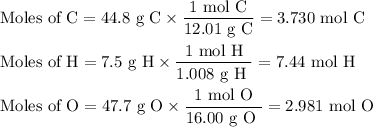Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, AIhunter2884
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2


Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Do you know the correct answer?
The sugar deoxyribose is an important component of DNA. Deoxyribose is 44.8% 7.5% H, and 47.7% O by...
Questions in other subjects:

Biology, 09.04.2020 03:12

Mathematics, 09.04.2020 03:12


Mathematics, 09.04.2020 03:12


English, 09.04.2020 03:12

English, 09.04.2020 03:12


English, 09.04.2020 03:12

Health, 09.04.2020 03:12