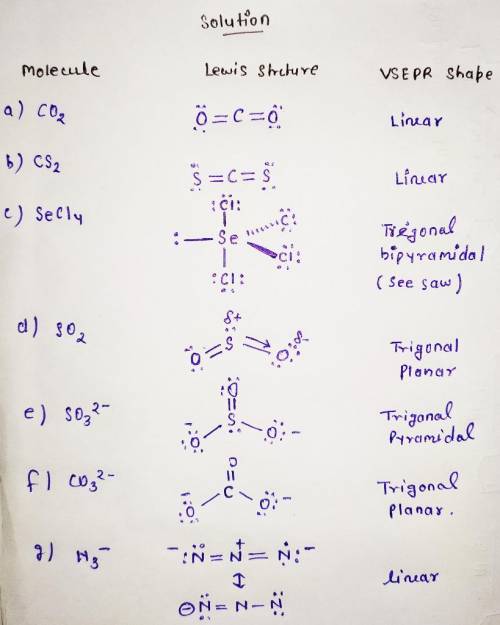
Sketch the simple Lewis dot structures and the predicted VSEPR
shapes for each of the followi...

Chemistry, 21.05.2020 00:10, Brainly264
Sketch the simple Lewis dot structures and the predicted VSEPR
shapes for each of the following species. Be sure to clearly indicate
lone pair electrons, multiple bonds (double or triple), and any three-
dimensionality (using dashes and wedges). For any ions, don’t forget
to account for the charge when calculating valence electrons! Only
expand the octet of the central atom when absolutely necessary.
A. CO2
B. CS2
C. SeCL
D. SO2
E. SO32-
F. CO32-
G. N3-

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:10, josephpezza18
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 05:50, mrylenastewart
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 07:30, kimberlyrios12p0ts98
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 12:30, quantamagic
Word equation for k(s)+h2o(l) yield koh(aq) + h2(g)
Answers: 1
Do you know the correct answer?
Questions in other subjects:



Mathematics, 27.09.2019 01:00

Biology, 27.09.2019 01:00


History, 27.09.2019 01:00

Geography, 27.09.2019 01:00



History, 27.09.2019 01:00