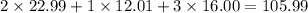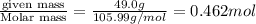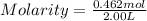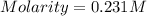Chemistry, 21.05.2020 00:12, lorenzo1beaton
What is the molarity if 2.00 liters containing 49.0 grams of sodium carbonate [Na2CO3)?
(Molar mass of Na is 22.99 g/mol, C is 12.01 g/mol and O is 16.00 g/mol.)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:10, josephpezza18
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1


Chemistry, 22.06.2019 19:00, andrecoral105
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 23.06.2019 03:00, sharondacarruth1656
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1
Do you know the correct answer?
What is the molarity if 2.00 liters containing 49.0 grams of sodium carbonate [Na2CO3)?
(Molar...
(Molar...
Questions in other subjects:

Mathematics, 08.07.2019 03:30


English, 08.07.2019 03:30

Chemistry, 08.07.2019 03:30





Physics, 08.07.2019 03:30


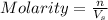
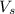 = volume of solution in L
= volume of solution in L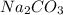 =
=