Chemistry, 19.05.2020 14:58, Demarcusclincy
Chloroform is a commonly used anesthetic with a density of 1.483 g/mL. Determine the volume of chloroform needed to deliver a 9.37 g sample of the anesthetic

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, sammiehammer
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2

Chemistry, 22.06.2019 12:50, khorasanpublic
The number at the end of an isotope’s name is the number.
Answers: 1


Chemistry, 23.06.2019 01:30, yarrito20011307
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
Do you know the correct answer?
Chloroform is a commonly used anesthetic with a density of 1.483 g/mL. Determine the volume of chlor...
Questions in other subjects:

Mathematics, 20.10.2020 01:01

Mathematics, 20.10.2020 01:01


Mathematics, 20.10.2020 01:01

Geography, 20.10.2020 01:01




Mathematics, 20.10.2020 01:01

English, 20.10.2020 01:01


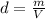 ⇒
⇒