

Answers: 3
Similar questions

Mathematics, 19.07.2019 00:20, reaunnatowns
Answers: 1

Chemistry, 03.08.2019 23:50, rgilliam3002
Answers: 1


Physics, 10.09.2019 23:30, wolfsaway
Answers: 3
Do you know the correct answer?
Write the complete name (prefix + unit) for each of the following numerical values:
a) 0.10 g<...
a) 0.10 g<...
Questions in other subjects:



Mathematics, 08.01.2020 06:31





Business, 08.01.2020 06:31

Mathematics, 08.01.2020 06:31


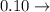 deci
deci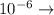 micro
micro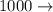 kilo
kilo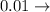 centi
centi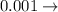 milli
milli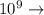 giga
giga




