Chemistry, 19.05.2020 03:17, Redhead667
1.What mass of oxygen gas, O2, from the air is consumed in the combustion of 702g of octane, C8H18, one of the principal components of gasoline? 2C8H18 + 25O2 -> 16CO2 + 18H2O.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:00, chrisxxxrv24
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 07:00, daniellekennedy05
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 10:30, angemango3423
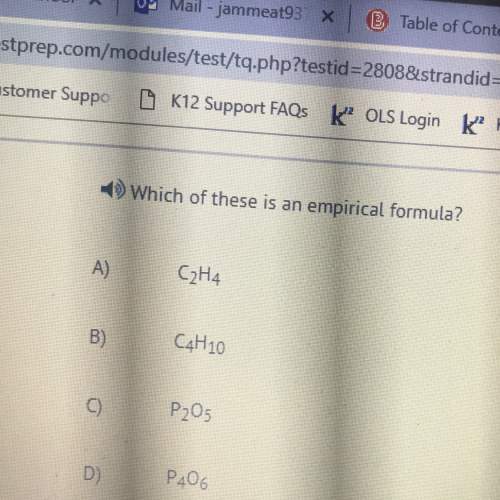
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1
Do you know the correct answer?
1.What mass of oxygen gas, O2, from the air is consumed in the combustion of 702g of octane, C8H18,...
Questions in other subjects:


History, 24.02.2021 22:00



Mathematics, 24.02.2021 22:00

Mathematics, 24.02.2021 22:00





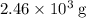 .
.  :
: 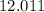 .
.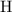 :
: 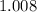 .
. :
: 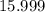 .
.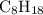 :
: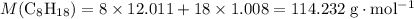 .
.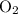 :
: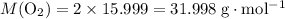 .
.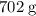 of octane,
of octane, 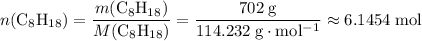 .
.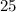 , whileThe coefficient of
, whileThe coefficient of 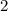 .
.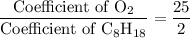 .
.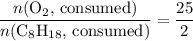 .
.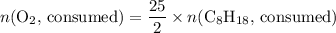 .
.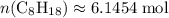 . Hence, the number of moles of oxygen gas required will be:
. Hence, the number of moles of oxygen gas required will be: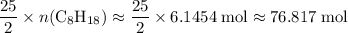 .
.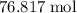 of
of 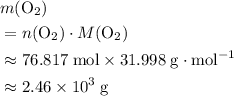 .
.