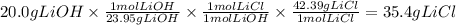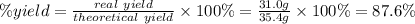Chemistry, 19.05.2020 02:15, jluckie080117
A reaction starts with 20.0 g of lithium hydroxide (LiOH) and actually produces 31.0 g of lithium chloride (LiCl), what is the percent yield? (Hint: First calculate the theoretical yield of lithium chloride (LiCl))
64.5%
88.6%
81.5%
92.8%


Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, jzjajsbdb8035
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 06:00, Chente379
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 06:20, Naysa150724
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 09:30, matpakootas521
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2
Do you know the correct answer?
A reaction starts with 20.0 g of lithium hydroxide (LiOH) and actually produces 31.0 g of lithium ch...
Questions in other subjects:

Chemistry, 05.02.2021 02:10

Mathematics, 05.02.2021 02:10


Health, 05.02.2021 02:10

Mathematics, 05.02.2021 02:10

Arts, 05.02.2021 02:10

Mathematics, 05.02.2021 02:10

History, 05.02.2021 02:10

Mathematics, 05.02.2021 02:10