Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, ayaanwaseem
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 20:30, lexibyrd120
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 22.06.2019 21:20, jordan2875
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1

Chemistry, 22.06.2019 22:30, darceline1574
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
Do you know the correct answer?
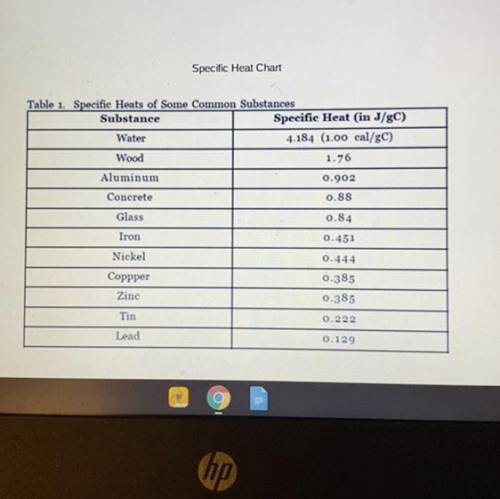
A copper penny with a mass of 35.4g is heated to 100.0˚C. It is then dropped into a cup of water wit...
Questions in other subjects:





Health, 24.08.2019 19:10

Biology, 24.08.2019 19:10

Mathematics, 24.08.2019 19:10

Mathematics, 24.08.2019 19:10

Computers and Technology, 24.08.2019 19:10