Chemistry, 07.05.2020 06:06, reneecoutinho10
The addition of 5.0x10–3 total moles of Zn2+ to a 1.0 L solution of NaCN gives a solution of the complex ion [Zn(CN)4] 2– (Kf = 4.17x1019). What is the equilibrium concentration of uncomplexed Zn2+ ions if the concentration of cyanide ions at equilibrium is 0.50 M?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:00, MilanPatel
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Do you know the correct answer?
The addition of 5.0x10–3 total moles of Zn2+ to a 1.0 L solution of NaCN gives a solution of the com...
Questions in other subjects:

History, 08.10.2019 04:10

History, 08.10.2019 04:10

Mathematics, 08.10.2019 04:10








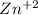 = 1.9 *
= 1.9 *