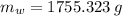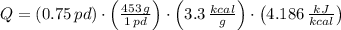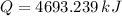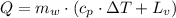Chemistry, 07.05.2020 05:12, marlesly87
The fuel value of hamburger is approximately 3.3 kcal/g. If a man eats 0.75 pounds of hamburger for lunch and none of the energy is stored in his body, estimate the amount of water that would have to be lost in perspiration to keep his body temperature constant. The heat of vaporization of water may be taken as 2.41 kJ/g.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, Sarahinator04
0.09 moles of sodium sulfate in 12 ml of solution
Answers: 3

Chemistry, 22.06.2019 17:30, rollercoasterbuddies
Why is the melting of ice a physical change ?
Answers: 1

Chemistry, 23.06.2019 00:20, HernanJe6
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 01:00, Johnson926
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2
Do you know the correct answer?
The fuel value of hamburger is approximately 3.3 kcal/g. If a man eats 0.75 pounds of hamburger for...
Questions in other subjects:

Mathematics, 21.01.2021 21:10


Advanced Placement (AP), 21.01.2021 21:10


Chemistry, 21.01.2021 21:10

Law, 21.01.2021 21:10

Mathematics, 21.01.2021 21:10


Mathematics, 21.01.2021 21:10

Mathematics, 21.01.2021 21:10


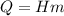
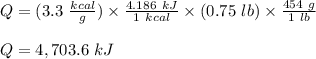
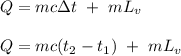
![Q = m[c(t_2 -t_1) \ + \ Lv]\\\\m = \frac{Q}{c(t_2 -t_1) \ + \ Lv} \\\\m = \frac{4,703.6 \times 10^3 }{4.186 (100-37) \ + \ 2.41 \times 10^3} \\\\m = 1,759.2 \ g\\\\m = 1.76 \ kg](/tpl/images/0651/6942/32919.png)