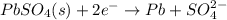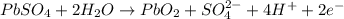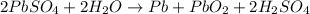Chemistry, 07.05.2020 05:08, ashiteru123
The lead-containing reactant(s) consumed during recharging of a lead-acid battery is/are . The lead-containing reactant(s) consumed during recharging of a lead-acid battery is/are . PbSO4 (s) only Pb (s) only PbO2 (s) only both PbO2 (s) and PbSO4 (s) both Pb (s) and PbO2 (s)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, braydentillery1221
If the particles in a sample of matter have an orderly arrangement and move only in place, the sample is a
Answers: 1

Chemistry, 22.06.2019 03:30, asianaenaeh
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 06:00, lindseyklewis1p56uvi
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 08:00, flakko1899
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1
Do you know the correct answer?
The lead-containing reactant(s) consumed during recharging of a lead-acid battery is/are . The lead-...
Questions in other subjects:



English, 12.04.2021 19:30



History, 12.04.2021 19:30

Mathematics, 12.04.2021 19:30

Physics, 12.04.2021 19:30



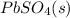
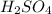 .
.