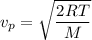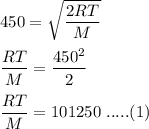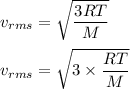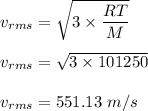Chemistry, 07.05.2020 04:58, justijust500
The most probable speed of an oxygen molecule in the gas phase at room temperature is 450 m/s. The root-mean-square speed (urms) is therefore equal to 450 m/s. much greater than 450 m/s. slightly less than 450 m/s. much less than 450 m/s. slightly greater than 450 m/s.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, wkalpakchi
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1


Chemistry, 22.06.2019 15:00, tcapele252
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Do you know the correct answer?
The most probable speed of an oxygen molecule in the gas phase at room temperature is 450 m/s. The r...
Questions in other subjects:


History, 17.02.2021 22:30

Biology, 17.02.2021 22:30

Mathematics, 17.02.2021 22:30


Chemistry, 17.02.2021 22:30

Mathematics, 17.02.2021 22:30



Mathematics, 17.02.2021 22:30