
Chemistry, 07.05.2020 04:58, anthonybowie99
Consider an electrochemical cell based on the spontaneous reaction 2AgCl(s) + Zn(s) → 2Ag(s) + 2Cl– + Zn2+. If the zinc ion concentration is kept constant at 1 M, and the chlorine ion concentration is decreased from 1 M to 0.001 M, the cell voltage should A) increase by 0.06 V. D) decrease by 0.18 V. B) increase by 0.18 V. E) increase by 0.35 V. C) decrease by 0.06 V.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:30, caeyanij
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 04:00, soonerlady19
Which atom or ion is the largest? 0 a. 0 0 0 0 e. li
Answers: 2

Do you know the correct answer?
Consider an electrochemical cell based on the spontaneous reaction 2AgCl(s) + Zn(s) → 2Ag(s) + 2Cl–...
Questions in other subjects:


Chemistry, 10.11.2020 01:10


Chemistry, 10.11.2020 01:10


English, 10.11.2020 01:10



Chemistry, 10.11.2020 01:10


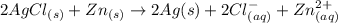
![E_{cell} = E^0 - \frac{0.059}{n} log [\frac{product}{reactant}]](/tpl/images/0651/5568/86cff.png)
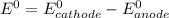
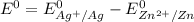
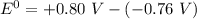
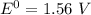
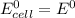 is as follows:
is as follows:![E_{cell} = 1.56 - \frac{0.059}{n} log [\frac{[Zn^{2+}]}{[Cl^-]^2}]](/tpl/images/0651/5568/33d2a.png)
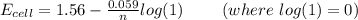
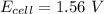
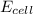 value in the decreased concentration of chlorine (aq) ion is calculated as:
value in the decreased concentration of chlorine (aq) ion is calculated as: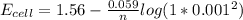
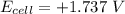
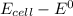
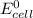 value after the decreased concentration of Chlorine is greater than the
value after the decreased concentration of Chlorine is greater than the 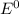 before the change; then there is increase in the value by 0.18 V
before the change; then there is increase in the value by 0.18 V



