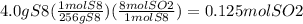Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 23:30, johnnysteeler9934
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1

Chemistry, 23.06.2019 03:00, kuehlthau03
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1

Chemistry, 23.06.2019 06:20, Lindsay882
Type the correct answer in each box. balance the chemical equation.__ n203 ➡️ __ n2 +__ o2
Answers: 1
Do you know the correct answer?
For the following equation; S8 + ___ O2 à ___ SO2 a. State what type of reaction is taking place? b...
Questions in other subjects:



History, 28.08.2020 23:01



History, 28.08.2020 23:01

Mathematics, 28.08.2020 23:01



Mathematics, 28.08.2020 23:01