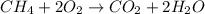Chemistry, 06.05.2020 23:07, Trucofer2106
Acetylene gas (C2H2) reacts with oxygen to produce carbon dioxide and water. When 30.0 g of acetylene is reacted with O2, 18.5 g of water is formed.
2 C2H2 (g) + 3 O2 (g) → 2 CO2 (g) + 2 H2O (l)
What type of reaction is this?
What is the percent yield of this reaction?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, celestemaria0727
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 14:50, chem1014
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2


Chemistry, 23.06.2019 00:30, tateandvioletAHS14AY
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1
Do you know the correct answer?
Acetylene gas (C2H2) reacts with oxygen to produce carbon dioxide and water. When 30.0 g of acetylen...
Questions in other subjects:








English, 18.03.2021 06:30

Mathematics, 18.03.2021 06:30