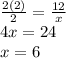Chemistry, 06.05.2020 21:33, richardnichol1111
According to the equation, 2 H2 + O2 --> 2 H20, how many moles of oxygen are required to convert 12 moles of hydrogen to water?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:10, krisandlance
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 22.06.2019 20:00, batoolishak7475
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
Do you know the correct answer?
According to the equation, 2 H2 + O2 --> 2 H20, how many moles of oxygen are required to convert...
Questions in other subjects:

Biology, 08.11.2020 16:30

History, 08.11.2020 16:30


Mathematics, 08.11.2020 16:30

Arts, 08.11.2020 16:30

Physics, 08.11.2020 16:30

English, 08.11.2020 16:30