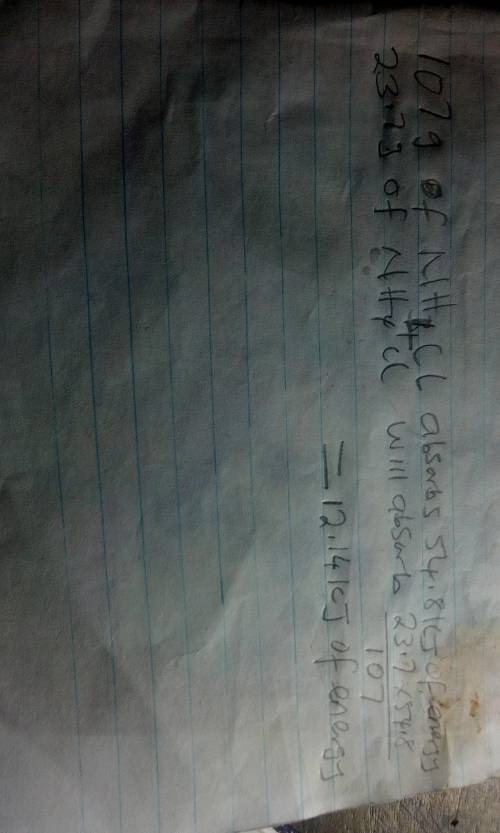Chemistry, 03.02.2020 20:45, PrincessIndia
The energy diagram shown represents the chemical reaction between solid ammonium chloride and solid barium hydroxide octahydrate:
2nh4cl(s) + ba(oh)2 x 8h2o(> 2nh3(aq)+bacl2(aq)+10h2o(1)
the (triangle then an h) for this reaction is 54.8 kj. how much energy would be absorbed if 23.7 g of nh4cl reacts?

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 05:30, sethjohnson386pbnm3x
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 05:30, madisonrosamond99
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2
Do you know the correct answer?
The energy diagram shown represents the chemical reaction between solid ammonium chloride and solid...
Questions in other subjects:

History, 02.11.2019 08:31





Biology, 02.11.2019 08:31


Mathematics, 02.11.2019 08:31


Mathematics, 02.11.2019 08:31