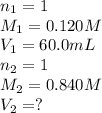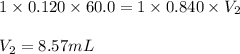Chemistry, 06.05.2020 20:11, vannybelly83
A volume of 60.0 mL of a 0.120 M HNO3 solution is titrated with 0.840 M KOH. Calculate the volume of KOH required to reach the equivalence point.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, vanessa051266
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 07:00, uniqueray33
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 23.06.2019 13:00, rachael382
How does the kinetic energy of a substance's particle in the solid phase compare to their kinetic enegy in the liquid phase?
Answers: 1
Do you know the correct answer?
A volume of 60.0 mL of a 0.120 M HNO3 solution is titrated with 0.840 M KOH. Calculate the volume of...
Questions in other subjects:


Mathematics, 06.03.2020 19:02

Mathematics, 06.03.2020 19:02

Business, 06.03.2020 19:02


Chemistry, 06.03.2020 19:02





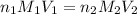
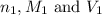 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 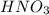
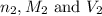 are the n-factor, molarity and volume of base which is KOH.
are the n-factor, molarity and volume of base which is KOH.