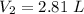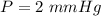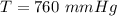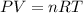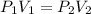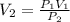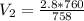After the end of a normal inspiration, the volume of air in the lungs is about 2.8 L. Normally quiet inspiration is driven by a pressure difference of about 2 mm Hg. The air in the lungs is at 37C and after normal expiration it is at atmospheric pressure. Quiet inspiration is driven by the expansion of the chest cavity by contraction of the diaphragm, which expands the air in the lungs. How much is the air expanded to produce an decrease of 2 mmHg in pressure

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 19:00, andrecoral105
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 23.06.2019 04:00, anonymous1813
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2
Do you know the correct answer?
After the end of a normal inspiration, the volume of air in the lungs is about 2.8 L. Normally quiet...
Questions in other subjects:

Social Studies, 14.02.2022 07:40




Mathematics, 14.02.2022 07:40




Biology, 14.02.2022 07:40