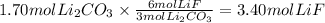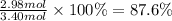The chemical reaction between lithium carbonate and aluminum trifluoride produces aluminum carbonate and lithium fluoride:
2AlF3 + 3Li2CO3 → Al2(CO3)3 + 6LiF.
You have an excess of aluminum trifluoride and 1.70 moles of lithium carbonate, which produces 2.98 moles of lithium fluoride. What is the percent yield of the reaction? Use the periodic table and this polyatomic ion resource.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, darkghostmist
What type of reaction fuels the processes seen here?
Answers: 2

Chemistry, 22.06.2019 17:20, alexis3060
How do you know when a chemical reaction has occurred
Answers: 1

Chemistry, 22.06.2019 21:00, itasykamila
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1
Do you know the correct answer?
The chemical reaction between lithium carbonate and aluminum trifluoride produces aluminum carbonate...
Questions in other subjects:


Mathematics, 01.03.2020 04:09


Mathematics, 01.03.2020 04:09

Mathematics, 01.03.2020 04:09

Spanish, 01.03.2020 04:09


Biology, 01.03.2020 04:10


Mathematics, 01.03.2020 04:10