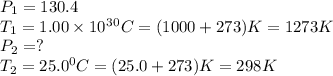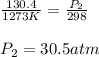Chemistry, 05.05.2020 21:33, caprisun6779
Calculate the final pressure inside a scuba tank after it cools from 1.00 x 103 °C to 25.0 °C. The initial pressure inside the tank was 130.4 atm.

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 18:00, rodriguezscarlet1713
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 22.06.2019 18:00, LuvieAnn1886
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3
Do you know the correct answer?
Calculate the final pressure inside a scuba tank after it cools from 1.00 x 103 °C to 25.0 °C. The i...
Questions in other subjects:










Mathematics, 09.02.2021 23:30


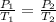
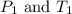 are the initial pressure and temperature of the gas.
are the initial pressure and temperature of the gas.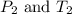 are the final pressure and temperature of the gas.
are the final pressure and temperature of the gas.