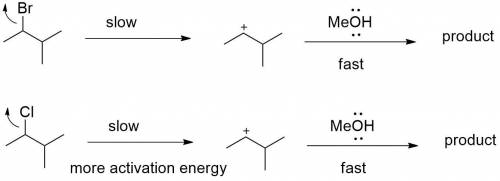
Chemistry, 05.05.2020 20:35, jacobp0712
Explain how the following changes would affect the rate of the reaction of 2-bromo-3-methylbutane with methanol: Part A The alkyl halide is changed to 2-chloro-3-methylbutane. The alkyl halide is changed to 2-chloro-3-methylbutane. The reaction will be slower because the leaving group will be poorer. The reaction will be faster because the leaving group will be better. The reaction will be slower because the leaving group will be better. The rate of the reaction does not change.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, dinosaur10
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 07:30, candigirl8847
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 11:00, jodonw5616
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 14:00, leahstubbs
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
Do you know the correct answer?
Explain how the following changes would affect the rate of the reaction of 2-bromo-3-methylbutane wi...
Questions in other subjects:


Business, 14.01.2021 20:20

Health, 14.01.2021 20:20


Social Studies, 14.01.2021 20:20

Arts, 14.01.2021 20:20



History, 14.01.2021 20:20

History, 14.01.2021 20:20


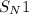 reaction.
reaction.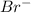 is a better leaving group than
is a better leaving group than 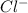 due to higher polarizability of
due to higher polarizability of