
Under certain conditions the rate of this reaction is zero order in ammonia with a rate constant of ·0.0038Ms−1: 2NH3(g)→N2(g)+3H2(g) Suppose a 450.mL flask is charged under these conditions with 150.mmol of ammonia. How much is left 20.s later? You may assume no other reaction is important. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:50, Catracho3619
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 21.06.2019 23:30, lylessd423
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2


Chemistry, 22.06.2019 21:40, fatherbamboo
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
Do you know the correct answer?
Under certain conditions the rate of this reaction is zero order in ammonia with a rate constant of...
Questions in other subjects:

Mathematics, 20.10.2020 04:01

History, 20.10.2020 04:01


Mathematics, 20.10.2020 04:01

Mathematics, 20.10.2020 04:01






 mmol of
mmol of 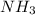 is left after 20 s.
is left after 20 s.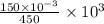 M = 0.333 M
M = 0.333 M![[NH_{3}]=-kt+[NH_{3}]_{0}](/tpl/images/0641/7445/9cd63.png)
![[NH_{3}]](/tpl/images/0641/7445/acd38.png) represents concentration of
represents concentration of ![[NH_{3}]_{0}](/tpl/images/0641/7445/b342c.png) is initial concentration of
is initial concentration of ![[NH_{3}]=(-0.0038M.s^{-1}\times 20s)+0.333M](/tpl/images/0641/7445/bbef3.png)
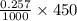 mol = 0.11565 mol
mol = 0.11565 mol



