
Chemistry, 05.05.2020 18:40, lillianneal
Substance ΔG°f(kJ/mol) M3O4(s) −8.80 M(s) 0 O2(g) 0 Consider the decomposition of a metal oxide to its elements, where M represents a generic metal. M3O4(s)↽−−⇀ 3M(s)+2O2(g) What is the standard change in Gibbs energy for the reaction, as written, in the forward direction? ΔG∘rxn= kJ/mol What is the equilibrium constant of this reaction, as written, in the forward direction at 298 K? K= What is the equilibrium pressure of O2(g) over M(s) at 298 K?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, cheyennecarrillo14
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1


Chemistry, 23.06.2019 01:30, Sonicawesomeness
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2
Do you know the correct answer?
Substance ΔG°f(kJ/mol) M3O4(s) −8.80 M(s) 0 O2(g) 0 Consider the decomposition of a metal oxide to i...
Questions in other subjects:


Mathematics, 12.01.2021 14:00


Chemistry, 12.01.2021 14:00



Mathematics, 12.01.2021 14:00

Chemistry, 12.01.2021 14:00


Social Studies, 12.01.2021 14:00


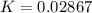
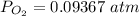
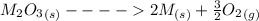
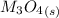 is
is 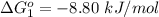
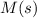 is
is 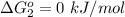
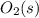 is
is 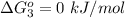
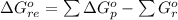
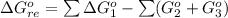
![\Delta G^o_{re} =[ (2 * 0) + (\frac{3}{2} * 0 )] - [1 * - 8.80]](/tpl/images/0641/3844/374c7.png)
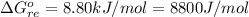

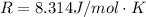
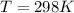
![K_p =[ P_{O_2}]^{\frac{3}{2} }](/tpl/images/0641/3844/66043.png)
![[ P_{O_2}]](/tpl/images/0641/3844/bc839.png) is the equilibrium pressure of oxygen
is the equilibrium pressure of oxygen 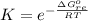
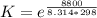
![0.02867 = [P_{O_2}]^{[\frac{3}{2} ]}](/tpl/images/0641/3844/d1a4b.png)
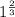
![P_{O_2} = [0.02867]^{\frac{2}{3} }](/tpl/images/0641/3844/dba69.png)




