Chemistry, 05.05.2020 16:42, caylah5921
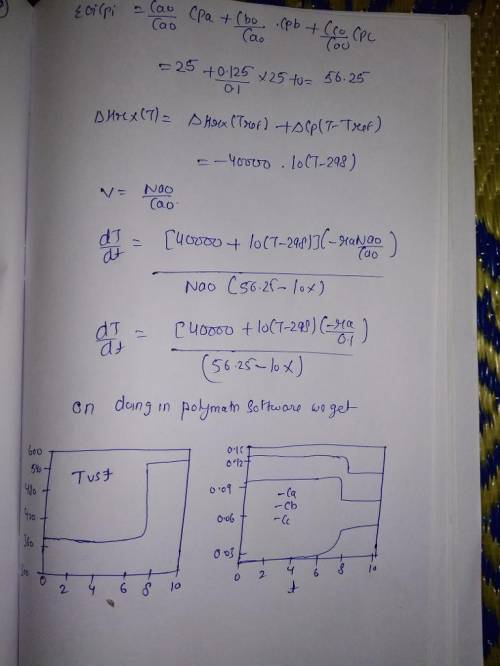
Is carried out adiabatically in a constant-volume batch reactor. The rate law is Plot and analyze the conversion, temperature, and concentrations of the reacting species as a function of time. Additional information: Initial Temperature 100C k1 (373 K) 2 103 s1 E1 100 kJ/mol k2 (373 K) 3 105 s1 E2 150 kJ/mol CA0 0.1 mol/dm3 25 J/mol K CB0 0.125 mol/dm3 25 J/mol K (298 K) 40,000 J/mol A 40 J/mol K

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, bibhu42kumarp7o4ss
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 23.06.2019 00:20, jessicamcummins
What type of context clue you understand the meaning of quandary?
Answers: 3

Chemistry, 23.06.2019 00:30, motorxr714
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1

Chemistry, 23.06.2019 01:00, davelopez979
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1
Do you know the correct answer?
Is carried out adiabatically in a constant-volume batch reactor. The rate law is Plot and analyze th...
Questions in other subjects:

History, 20.08.2019 12:30

Mathematics, 20.08.2019 12:30

Mathematics, 20.08.2019 12:30

Social Studies, 20.08.2019 12:30


Geography, 20.08.2019 12:30




English, 20.08.2019 12:30