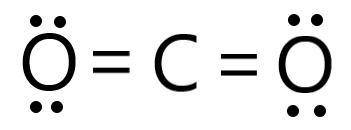Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, makenziehook8
Which is true of a liquid? it has a definite volume but not a definite mass. it has a definite mass but not a definite volume. it has a definite volume but not a definite shape. it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 13:50, awesomegamergurl13
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2
Do you know the correct answer?
Determine the electron geometry (eg) and molecular geometry (mg) of CO2.
eg=trigonal planar, mg...
eg=trigonal planar, mg...
Questions in other subjects:



Physics, 17.10.2019 07:50


Mathematics, 17.10.2019 07:50


Mathematics, 17.10.2019 07:50

History, 17.10.2019 07:50

Spanish, 17.10.2019 07:50

Mathematics, 17.10.2019 07:50