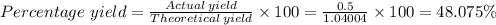100.0mL of 0.40M Al(NO3)3 is reacted with 100.0 mL of 0.40M Al(OH)3 according to
the following reaction: Al(NO3)3 + 3KOH → Al(OH)3 + 3KNO3
1) Identify the limiting reactant.
2) Identify the excess reactant.
3) Determine the theoretical yield of Al(OH)3
4) How much excess reactant is left over after the reaction is complete?
5) If 0.50g of Al(OH)3 determine the percent yield of Al(OH)3

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, cheesecake1919
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 16:00, esme06quirino
Is a measure of the resistance to flow. a high liquid has a high resistance to flow and flows slowly. the ancients thought everything in the world was made of 4 we now know that there are 94 naturally occurring and scientists have created another 24 i am certain they will create even more. honey flows slowly because it has a high to flowing. a can be separated by physical means because it contains more than one pure substance and 2 pure substances are not chemically bonded to each other. a cannot be separated by physical means. all matter is made up of all elements are with the same number of protons. if it is just a single or many bonded together, if all of them have the same number of protons, it is an element. in a piece of pure iron metal, all the are joined together, that piece of iron metal is called elemental iron. a single of iron is called elemental iron. a mixture has differences from place to place. we might need a microscope to see them or they might be obvious to the unaided eye. there are surfaces separating it into different phases. a mixture is the same everywhere. it is uniform. there are no surfaces separating it into different phases. if different kinds of atoms (different elements) are bonded together by their electrons, it is called a there are physical means of to isolate the different pure substances in a mixture and there are chemical means of to isolate the different elements in a compound. 1. element 2. compound 3. mixture 4. heterogeneous 5. homogeneous 6. pure substance 7. atoms 8. separation 9. viscosity 10. resistance
Answers: 2

Chemistry, 22.06.2019 23:30, sanociahnoel
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
Do you know the correct answer?
100.0mL of 0.40M Al(NO3)3 is reacted with 100.0 mL of 0.40M Al(OH)3 according to
the following...
the following...
Questions in other subjects:

English, 15.07.2021 17:10


Computers and Technology, 15.07.2021 17:10


Mathematics, 15.07.2021 17:10

Arts, 15.07.2021 17:10

Physics, 15.07.2021 17:10

Arts, 15.07.2021 17:10

Chemistry, 15.07.2021 17:10