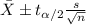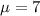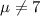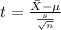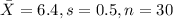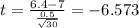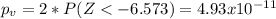Chemistry, 04.05.2020 22:37, lydia1melton
The Environmental Protection Agency has determined that safe drinking
water should have an average pH of 7 moles per liter. You are testing water from a new source, and take 30 vials of water. Water is unsafe if it deviates too far from 7 moles per liter in either direction. The mean pH level in your sample is 6.4 moles per liter, which is slightly acidic. The standard deviation of the sample is 0.5 moles per liter.
b) A 95% confidence interval for the true mean pH level of the water is (6.21, 6.59). Interpret this interval.
c) Explain why the interval in part (b) is consistent with the result of the test in part (a).

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:50, nnaomii
What is the composition, in atom percent, of an alloy that consists of 4.5 wt% pb and 95.5 wt% sn? the atomic weights for pb and sn are 207.19 g/mol and 118.71 g/mol, respectively.(a) 2.6 at% pb and 97.4 at% sn(b) 7.6 at% pb and 92.4 at% sn(c)97.4 at% pb and 2.6 at% sn(d) 92.4 at% pb and 7.6 at% sn
Answers: 2

Chemistry, 21.06.2019 19:30, genyjoannerubiera
Si una estrella no tiene paralaje medible, ¿qué puedes inferir?
Answers: 1


Chemistry, 22.06.2019 16:30, joshua1255
Find the number of moles of argon in 364g of argon.
Answers: 2
Do you know the correct answer?
The Environmental Protection Agency has determined that safe drinking
water should have an ave...
water should have an ave...
Questions in other subjects:

Mathematics, 19.04.2021 18:20

Spanish, 19.04.2021 18:20




Mathematics, 19.04.2021 18:20



Chemistry, 19.04.2021 18:20