
Chemistry, 03.05.2020 13:04, Kaziyah461
0.80 mol MgBr2 is added to 1.00 kg water. Determine the freezing point of the solution. Water has a
freezing point depression constant of 1.86°C. kg/mol.
Which equation should you use?
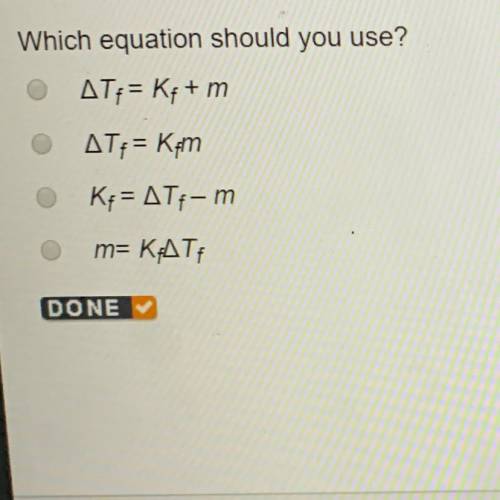

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:40, kellypechacekoyc1b3
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 14:30, neidaq12345
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 22.06.2019 16:50, lilblackbird4
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 16:50, TheOriginal2x
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3
Do you know the correct answer?
0.80 mol MgBr2 is added to 1.00 kg water. Determine the freezing point of the solution. Water has a<...
Questions in other subjects:








Biology, 31.07.2019 17:00

Social Studies, 31.07.2019 17:00







